Bridging the gap between buckyballs and graphene
(Nanowerk News) The discovery of the soccer-ball-shaped molecule ‘buckminsterfullerene’ (or buckyball) in 1985 kick-started a revolution in carbon nanomaterials that continues today with research into extremely conductive graphene. Now, an all-RIKEN team has used the institution’s K computer to predict the stability of structures that bridge the gap between these two extremes, namely individual fullerenes and infinite sheets of carbon (" From C60 to infinity: large-scale quantum chemistry calculations of the heats of formation of higher fullerenes").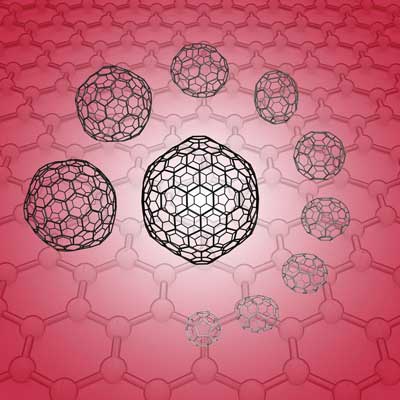
A RIKEN team has developed a computational method that allows them to determine the stability of carbon nanostructures lying between fullerene and infinite sheets of graphene. (© ACS)
The heat of formation is a fundamental property used by chemists to gauge the energy released or consumed during a reaction. For small compounds, it can be determined quite accurately using calorimeters. However, measurement errors generally increase with increasing molecular size, making it challenging to quantify the energy required to make fullerene (C60) from 60 carbon atoms.
State-of-the-art theoretical calculations have helped refine estimates of C60’s heat of formation, but questions exist about the uncertainties of these methods. Furthermore, analyzing more complex fullerenes requires supercomputers hundreds of times more powerful than those commonly available.
Kimihiko Hirao and colleagues from the RIKEN Advanced Institute for Computational Science realized that a change of approach might help crack this problem.
“Chemists consider a single molecule with hundreds of atoms very large,” says Hirao. “But to materials scientists, it would be one of the smallest known nanomaterials. Finding a way to bridge the molecular and materials worlds required us to step out of our usual mindset.”
The researchers first devised a series of model chemical reactions describing how the gradual build-up of carbon atoms into larger complexes can be represented by the formation of simple benzene rings. Similarly, they modeled the formation of oversized fullerenes in terms of a smaller, C20-based ring system. To develop procedures for rapidly analyzing large molecules, they used high-level supercomputer algorithms to evaluate the resulting heats of formation, as well as less computationally intense methods.
The quantum calculations determined the heat of formation of C60 with an uncertainty five times better than typical experiments. Additionally, the team saw that the per-atom heats of formation converged to a simple equation—enabling them to predict the existence of new fullerenes many times larger than C60. Intriguingly, this equation estimates that at least 10,000 carbons are needed to reduce bond strain in fullerenes and impart them with graphene-like properties.
“The number of differently sized fullerenes that exist between the extremes of C60 and graphene suggests potential for a vast range of applications,” says Hirao. “And the chemical and computational frameworks we developed can be easily used to compute other types of properties for large, but finite, molecules.”