Carbyne: twice the strength of Graphene?
The weird and wonderful world of nano-materials is gathering pace with many showing genuine promise. But while graphene has been seen as king, has it now been usurped?
In April this year researchers at the University of Vienna published findings in Nature Materials that caused quite a stir among organic chemists, and even the mainstream press.
The Vienna researchers have developed a method for producing linear chains of single carbon atoms that, at 6000 carbon atoms in length, are two orders of magnitude longer than had been previously possible.
Queue reports that carbyne – the missing one dimensional allotrope of carbon – had been produced in bulk for the first time. But many of these reports were not entirely accurate.
Rather, the University of Vienna work represents a ‘route to carbyne’, according to the senior author of the Nature Materials paper Thomas Pichler.
He says: “By definition, carbyne is an infinitely long chain of carbon atoms. As a solid state physicist, I call anything infinite if its properties do not change anymore by adding extra atoms. I would call it carbyne, but of course you can be very picky and say ‘it’s not infinitely long’.”
The excitement that Pichler and his team have generated is justified however. According to theoretical models, carbyne’s mechanical properties exceed those of all known materials, outperforming both graphene and diamond.
Rice University theoretical physicist Boris Yakobson says: “I came to think that modern theoretical/computational tools have reached the level when we can reliably predict carbyne properties prior to its actual availability – and this resulted in some quite remarkable predictions.”
According to calculations made by Yakobson and his group, carbyne’s tensile strength is double that of graphene. It also demonstrates twice the tensile stiffness of graphene and carbon nanotubes, and nearly three times that of diamond.
Stretching carbyne by as little as 10% alters its electronic band gap significantly, and if outfitted with molecular handles at its ends, it can also be twisted to alter its band gap. With a 90° end-to-end rotation, it becomes a magnetic semiconductor.
Further, carbyne chains can take on side molecules that may make them suitable for energy storage.
Yakobson explains: “The sp-hybridised carbon constrained in a linear structure makes it form either single-triple alternative bonds or double-double bonds. This structural variation enables it to have different electrical properties – semiconducting or metallic – and the sheer bond strength endows it with exceptional mechanical properties.”
The existence of carbyne was first proposed in 1885 by Adolf von Baeyer, who stated that the production of the material would remain elusive as its high reactivity would always lead to its immediate destruction.
Since then, numerous claims to the production of carbyne have been made and each has met with controversy owing to the slightly woolly definitions of what carbyne actually is. Most recently, researchers at Sun Yat-sen University claimed to have produced crystals of the material using pulsed laser radiation in a paper published in Science Advances.
However, Pichler says that this material isn't carbyne in the strictest sense.He explains: “They called it carbyne with finite-length, which is per definition not carbine as an infinite one-dimensional carbon chain. In contrast these samples contain a three-dimensional ensemble of short polyyne chains of a few atoms in length.
“These are two different things that in the literature are sometimes referred to by the same name.”
Semantics aside, Pichler’s team has shown that long chains of carbon atoms can be produced and can be stabilised. It is proof that the material can exist.
He says that fresh eyes were key to solving the problem: “I’m not an organic chemist, I’m from a completely different field and with a different approach.”
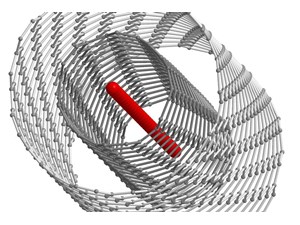
Pichler’s trick to stabilising the material is to grow it inside a double-walled carbon nanotube (DWCNT) with an inner wall of the right diameter, which provides uniform stabilization along the entire length of the carbon chain.
Pichler says: “You need to find a carbon nanotube with a suitable inner diameter to grow the ultra- long chains, and you have to use DWCNTs because single-walled carbon nanotubes are not stable at high temperatures. Multi-walled carbon nanotubes have been shown to work for the growth of short chains, but their inner diameters are not as suitable for the bulk production of ultra-long chains.”
These DWCNTs are synthesised by high-vacuum alcohol chemical vapour deposition from a catalyst. Typically, 0.5 g of catalyst powder yields tens of milligrammes of DWCNTs after purification. A thin buckypaper is prepared from these DWCNTs by rinsing, filtering and drying.
To produce linear carbon chains within these DWCNTs, the buckypapers are annealed under vacuum at high temperatures.
In collaboration with groups at the AIST Tsukuba in Japan, the ETH Zürich in Switzerland, the MPI Hamburg in Germany and UPV/EHU San Sebastian in Spain, the existence of the chains within these DWCNTs has been confirmed unambiguously using a multitude of complementary methods.
These include temperature dependent near- and far-field Raman spectroscopy with different lasers for the investigation of electronic and vibrational properties, high resolution transmission electron spectroscopy for the direct observation of carbyne inside the carbon nanotubes and x-ray scattering for the confirmation of bulk chain growth.
Pichler and his group will now focus on characterising the properties of the long carbon chains. The researchers have already noticed that their presence dramatically increases the photoluminescence of the inner tubes of the host DWCNTs, which could prove useful for biotechnology applications.
The team is also working on methods of extracting the carbon chains from the DWCNTs.
It could be many years before carbyne finds any industrial applications; one only has to look at the time it has taken to commercialise more stable carbon-based materials such as fullerenes, carbon nanotubes or graphene to appreciate the amount of work that is yet to be done.
However, Pichler's work represents some exciting first steps.
Yakobson concludes: “It opens the possibility of the application of these promising materials as well as understanding the fundamental physics. The impact ultimately will depend on any unusual properties that such a material can offer. But synthesizing the longest carbyne chains with DWCNT protection is quite a stunt in itself.”