Scientists Use Computer Simulations to Develop Graphene-Like Crystals of Salts
New computer simulations have explored the possibility that common rock salt (NaCl) may form graphene-like 2D crystals, a possiblity which has been theorized for some time.
The researchers developed an equation based on computer simulations that help determine how many layers in a salt crystal are required for it to break down into 2D layers. These ultrathin crystals could be used in nanelectronics.
The international research team, from Skolkovo Institute of Science and Technology (Skoltech), the National University of Science and Technology MISiS (Russia), Moscow Institute of Physics and Technology (MIPT), Rice University (USA), and the Technological Institute for Superhard and Novel Carbon Materials (TISNCM), published their work in theJournal of Physical Chemistry Letters.
From 3D to 2D
Graphene is formed of sheets just a single atom thick, giving it an array of unusual properties which make it very interesting to materials science researchers and product developers.
As graphene is effectively a single layer of graphite crystal, its properties, like that of other 2D crystals, differ from its 3D counterparts. A lot of research has been conducted to explore new 2D materials with extraordinary properties,after graphene was discovered.
Ultrathin films can be used in various micro- and nanoelectronic applications, because of their unique properties.
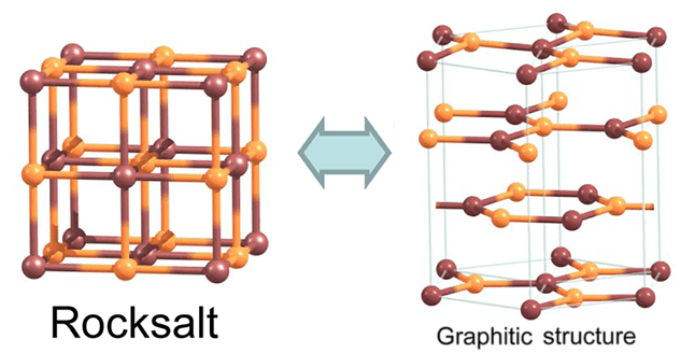
Theoretical research conducted in the past show that cube-structured films with ionic bonding can transform into a layered hexagonal graphitic structure through a process called graphitization. The conversion has been recorded during experiments, in the case of certain substances.
Rock salt, NaCl, has been predicted to have graphitization tendencies in the past. If cubic compounds are graphitized it can lead to the formation of promising, new structures that can be used in nanoelectronic applications.
Conversely, no theory exists to explain the process for, say, an arbitrary cube compound and can predict its conversion into graphene-like salt layers.
The number of crystal layers along the major diagonal of the cube will have to be decreased for the graphitization to take place. This will lead to the formation of two crystal surfaces, one with sodium ions Na+ and the other with chloride ions Cl-.
More importantly, the structure’s lattice points should be made of positive and negative ions (i.e. Na+ and Cl-), rather than neutral ones. As a result, charges of two opposing sign are formed on the surfaces. The charges cancel each other when the surfaces are at a distance and the salt slabs prefer to remain in a cubic structure.
However, a big dipole moment arises if the films are thin enough, because of the opposite charges in the crystal surfaces. The structure tries to thwart the dipole moment and thereby increases the system’s energy. A rearrangement of atoms takes place in the crystal structure in order to neutralize the surface charge.
Experiment vs. model
The researchers experimented with 16 binary compounds that have the common AB formula, with the aim of understanding how graphitisation tendencies differ from compound to compound.
The A in the formula represents one of the alkali metals including sodium Na, lithium Li, rubidium Rb, and potassium K. These metals fall in the Group 1 of the periodic table and are highly reactive in nature.
B represents an element from the halogen group, which includes chlorine Cl, iodine I, bromine Br, and fluorine F. These elements react well with alkali metals and are found under Group 17 in the periodic table.
The compounds used in the research fall under a variety of structures, called as phases or crystal lattices. An increase in the atmospheric pressure to 300,000 times its general amount leads to the stabilization of the B2 phase of NaCl.
The scientists stimulated two different crystal lattices, measuring the pressure that suits the phase transition between them, so as to test the parameters and techniques they employed. The experimental data was in accordance with their prediction.
Just How Thin Should it be?
All the compounds within the scope of the research have the potential to have hexagonal, “graphite” G phase, that can become the most stable structure when it comes to ultrathin films including quasi-2D and 2D films, while remaining unstable in 3D bulk.
The scientists found the relationship between the various layers of a film and its surface energy in both hexagonal and cubic structures.
They recorded this connection in the form of a graph representing all the compounds explored in the research using two lines, with varying slopes.
The pair of lines representing each compound meets at a particular point that matches the critical slab thickness which is energetically suited for the conversion of the structure from a cube to a hexagon. For instance, 19 to 27 layers were required in lithium salts and nearly 11 layers were required in all sodium salts.
The researchers were able to find a connection between the critical number of layers and two criteria that determine the ionic bonds’ strength in the compounds. The ionic radius or the size of a metal’s ion is the first criterion.
The electronegativity or the ability of atom A to draw the electrons of element B, is the second criterion. If the electronegativity is high the bond has a greater ionic nature, the ability of the atoms to attract the electrons is more, the surface dipole is bigger and the critical slab thickness is less.
And There’s More
The head of the Laboratory of New Materials Simulation at TISNCM, Pavel Sorokin, Dr. habil., talks about the significance of the research:
"This work has already attracted our colleagues from Israel and Japan. If they confirm our findings experimentally, this phenomenon [of graphitisation] will provide a viable route to the synthesis of ultrathin films with potential applications in nano electronics".
The researchers plan on exploring other compounds, expanding the scope of their research. They think spontaneous graphitization can occur in ultrathin films of various compositions, leading to the formation of new layered structures that have more interesting properties.